Trending Now
- 830 voters names go missing in Kavundampalayam constituency
- If BJP comes to power we shall consider bringing back electoral bonds: Nirmala Sitaraman
- Monitoring at check posts between Kerala and TN intensified as bird flu gets virulent in Kerala
Coimbatore
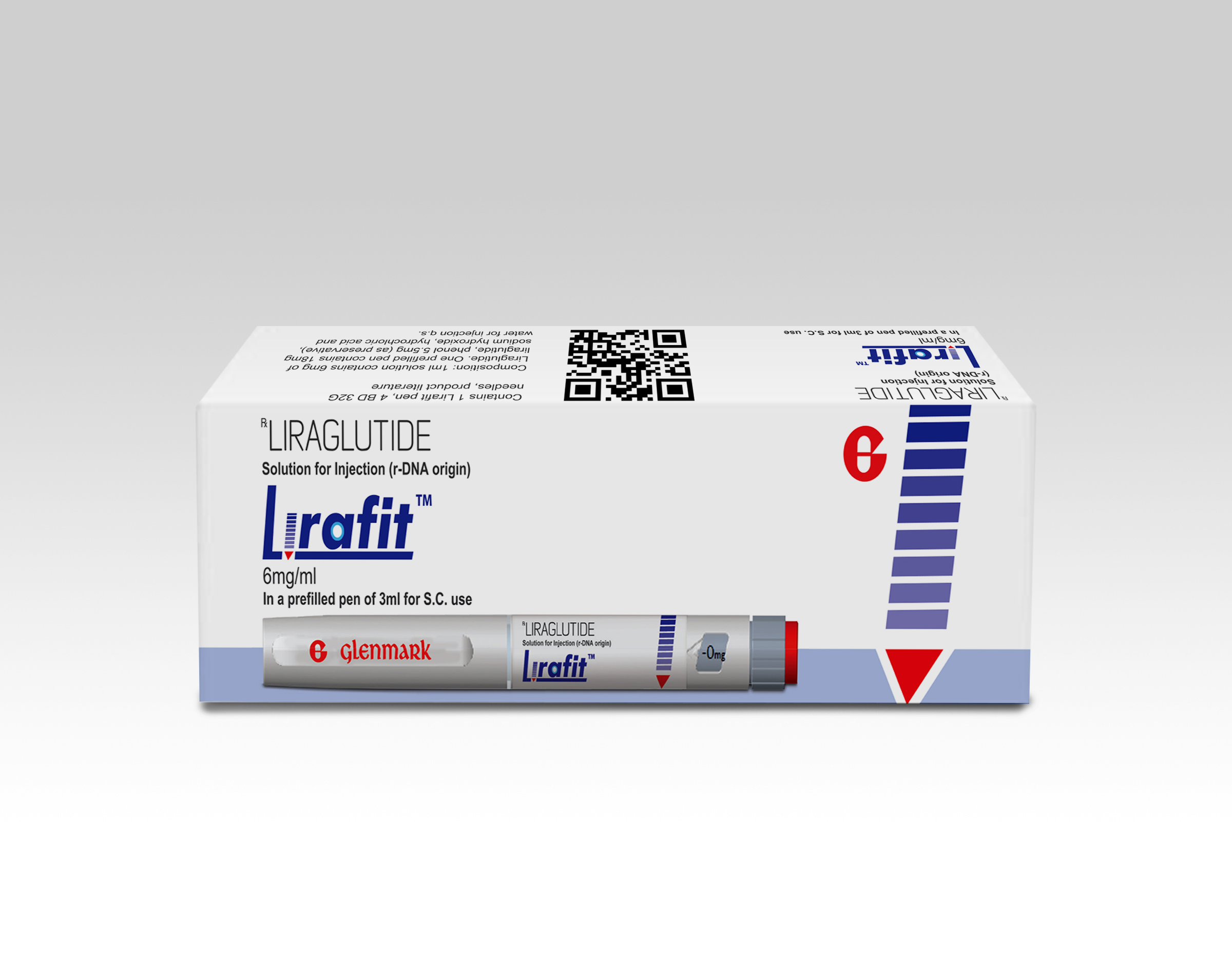
Glenmark is the first to launch Biosimilar of Popular Anti-Diabetic Drug, Liraglutide, in India
![]() January 4, 2024
January 4, 2024
Coimbatore : Glenmark Pharmaceuticals Ltd. (Glenmark), a research-led, global pharmaceutical company, has launched a biosimilar of the popular10,11 anti-diabetic drug, Liraglutide, for the first time in India. The drug is being marketed under the brand name Lirafit™ following the approval from the Drug Controller General of India (DCGI). Priced at around INR 100 for a standard dose of 1.2 mg (per day), this will lower the cost of therapy by approximately 70%, and will be available only under prescription.
Liraglutide belongs to the class of glucagon-like peptide 1 receptor agonist (GLP-1 RA) drugs, which increase glucose-dependent insulin secretion and decrease in appropriate glucagon secretion.12,13 It has been approved globally for the management of type 2 diabetes mellitus in adult patients in the United States and the European Union.14
“Glenmark is proud to introduce Lirafit™, a novel and affordable biosimilar of the drug liraglutide, for the first time in India. Clinical trials have shown that it helps improve glycemic control in adult type 2 diabetes mellitus patients along with atherosclerotic cardiovascular diseases (ASCVD) and obesity.1-5 Liraglutide has also proven to have a positive impact on cardiac and renal safety outcomes among patients in clinical trials, making it an effective choice of treatment for patients with type 2 diabetes mellitus.4,5,7,14,15 With this launch, we have now ventured into the injectable anti-diabetic market taking another significant stride in the diabetes therapy space,” remarked Alok Malik, President and Business Head ‐ India Formulations, Glenmark Pharmaceuticals Ltd.
Liraglutide and its role in the treatment of type 2 diabetes
Liraglutide has a proven efficacy in improving glycemic control in patients with type 2 diabetes mellitus. Clinical trials on Indian adult patients with type 2 diabetes mellitus over a 24-week period have demonstrated Lirafit™ to be effective, safe and well-tolerated. The trials also revealed non-inferior efficacy and a safety profile with that of the reference liraglutide.16 Additional benefits of liraglutide include effectively lowering glycemic parameters, weight reduction, and cardiovascular safety in patients with type 2 diabetes mellitus2-5,13,14.
GLP-1 RA class of drugs and their mechanism of action
GLP-1 RA (Glucagon-like peptide-1 receptor agonists) are a group of drugs used in the treatment of type 2 diabetes.13 GLP-1 RAs are very effective in lowering blood sugar levels. Several trials have demonstrated that GLP-1 RAs reduce cardiovascular risk in patients with type 2 diabetes mellitus and ASCVD or high cardiovascular risk and have beneficial effects on cardio-renal outcomes beyond their blood glucose-lowering effects in type 2 diabetes mellitus patients.17,18 Their mechanism of action involves the release of insulin, in the presence of elevated glucose concentrations, thus decreasing glucagon secretion.12,13 GLP-1 RAs are recommended in the treatment guidelines by the American Diabetes Association as well as the American Association of Clinical Endocrinology (AACE) Consensus Statement & European Society of Cardiology for type 2 diabetes mellitus patients with co-morbidities, like established atherosclerotic cardiovascular disease and obesity.6,7,8,9. The American Diabetes Association (ADA) also recommends GLP1RAs therapy for weight loss & lesser risk of hypoglycemia in type 2 diabetes mellitus patients.6,7 Drugs belonging to this class include liraglutide, semaglutide, and dulaglutide, among others.